Below is our recent interview with Andy Coravos, the Founder & CEO at HumanFirst:

Q: Could you provide our readers with a brief introduction to your company?
A: HumanFirst enables safe, effective, and equitable healthcare operations at home. Previously known as Elektra Labs, HumanFirst serves leading organizations pioneering decentralized clinical trials and virtual care. Twenty-two of the 25 largest pharmaceutical companies in the world have used the HumanFirst platform to evaluate and enable the deployment of health technology products. HumanFirst offers command center and infrastructure solutions that ensure their telehealth operations are as reliable and trustworthy as those within hospitals or labs.
HumanFirst was developed via grants from the National Science Foundation and the Harvard Business School Rock Center for Entrepreneurship, and first launched its Atlas workflow management tool for remote monitoring in 2019.
In September 2020, HumanFirst worked with leaders from the Digital Medicine Society, Genentech, a member of the Roche Group, Koneksa, Myokardia, Sage Bionetworks and Scripps Research to spearhead the creation of ‘The Digital Measures Playbook’ of best practices for capturing patient signals and decreasing risk when deploying, managing, and monitoring connected products in remote settings.
Q: Any highlights on your recent announcement?
A: HumanFirst closed a Series A round of funding led by Maverick Ventures, participation from Lux Capital, Threshold Ventures, Arkitekt Ventures, Boost VC, SV Angel, Village Global, and more than 30 angel investors. This investment brings HumanFirst’s total funding to $15M since the company’s inception.
Recommended: 11 Things Employees Should Know About Returning To The Workplace
Q: Can you give us more insights into your offering?
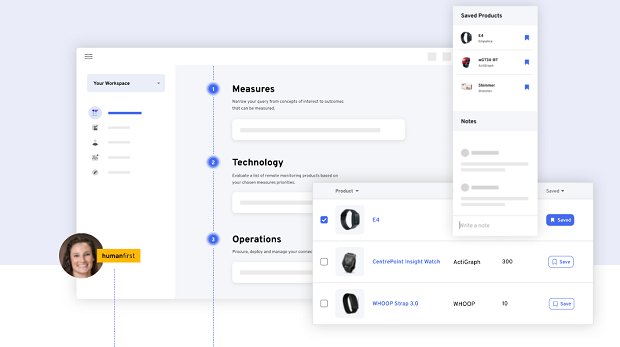
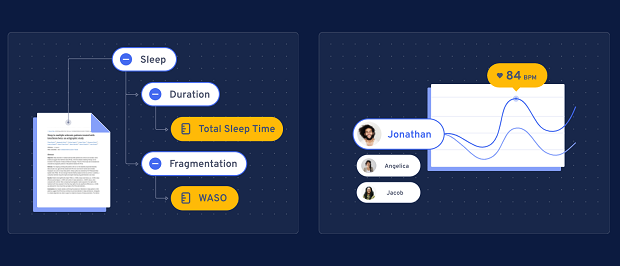
A: Today, our customers can use our Atlas Platform’s workflow tools to avoid common pain points when looking to rapidly deploy remote monitoring. Drawing from The Playbook, an open-access resource governed by the Digital Medicine Society (DiMe), our tools follow a three step process: measures, technologies and operations. In Step 1 (Measures), the Atlas platform supports researchers and providers to build an evidence base for the concepts they want to measure (e.g.,sleep) and drills into the most researched measures (e.g., wake after sleep onset) in a given population. After building an evidence base for measures, Step 2 (Technology) makes it easy to look at a product’s evidence (peer reviewed papers, clinical trials), utility/usability, security features, data governance, and economic considerations. We’re using this next round of funding to support more of the fulfilment, at-home distribution of sensors, which is Step 3 (Operations) via The Playbook.
Use case 1 – Deploying digital measures in an upcoming study: In the past year our primary customer has been pharma sponsors who have had to shift their trials to the home. Most often we work with study teams who believe a decentralized trial approaches could:
speed up the study,
reduce participant burden, and
provide more valuable and patient-centric information.
For example, a team believes that a connected sensor is the best way to measure what matters to patients.They want to better understand how their drug impacts quality of life metrics such as sleep and activity. The team has questions such as: What’s the best way to measure sleep? Which wearables can accurately measure sleep? Which sensors have been successfully used in a clinical trial already? The interactive measures and technology reports provided through the Atlas platform enable these questions to be answered.
Our team at HumanFirst published some example case studies via the open-access Playbook, and also related use cases on using digital measures in research have been published within Clinical Trial Transformation Initiative (CTTI), a public-privacy partnership between Duke and the FDA.
Use Case 2 – Conducting a landscape analysis/portfolio strategy for a specific therapeutic area to understand what digital measures have been well studied in a particular area (i.e Immunology or Neurology). In these instances, our customers will run hypotheses and develop queries using the Atlas dataset to identify therapeutic areas and also technologies that are most amenable to digital measures.
Use Case 3 (Mentioned above) – Cataloging digital measures and technologies previously deployed within the organization. Since the pandemic hit, the adoption of digital endpoints has increased 5x during the pandemic (source), many teams are struggling to understand what measures and technologies have been deployed across their organizations. We have an API-driven platform that makes it easy to track sensor deployments — and integrate it into bigger systems when needed.
Q: What can we expect from your company in next 6 months? What are your plans?
A: With our new Series A funding, we will expand to enable an API-led operational infrastructure related to decentralized trials and distributed care. Customers will be able to choose the Atlas API for customer solutions and the ability to ingest metadata from 3000+ measures and 1000+ sensors into their own apps.
We are also expanding into new product offerings, like a historical tech catalog to become the single source of truth for an organization’s use of remote monitoring. Since the pandemic hit, the adoption of digital endpoints has increased 5x during the pandemic (source), many teams are struggling to understand what measures and technologies have been deployed across their organizations. We have an API-driven platform that makes it easy to track sensor deployments — and integrate it into bigger systems when needed.
Lastly, we are hiring to grow our team. We are actively hiring product, engineering, operations staff. Join us to bring healthcare home!
Q: What is a great thing about your company that people might not know about?
A: HumanFirst offers comprehensive data workflow so you can sift through 1000+ connected sensor technologies, 3000+ digital clinical measures classified in 150+ categories, 300+ medical conditions spanning 25+ therapeutic areas.